First of all determine the valence electron that is available for drawing the lewis structure of SeF4 because the lewis diagram is all about the representation of valence electrons on atoms. Count total valence electron in HClO3.

How To Draw Lewis Structures A Step By Step Tutorial Middle School Science Blog
Count total valence electron in SeF4.

. For the BF 3 Lewis structure calculate the total number of valence electrons for the BF 3 molecule. There are a total of 24 valence electrons for the BF 3 Lewis structure. The formal charge can be calculated by using the formula- Steps for Drawing Lewis Structure of IF3.
Follow some steps for drawing the Lewis structure for HClO3. Drawing the Lewis Structure for PH 3. The best Lewis structure for any molecule should have each atom with a formal charge of 0.
This further helps in understanding how the atoms bond the physical properties of the molecule and it also plays an important role in the way that biological molecules interact with each other. A video tutorial for how to draw Lewis Structures in five steps. For the PH3 Lewis structure we first count the valence electrons for the PH3 molecule using the periodic table.
Let us start by counting the total number of. The central atom of the HClO3 lewis structure violates the octet rule as it contains more than 8 electrons around it. Steps for drawing Lewis Dot Structure.
Drawing the Lewis Structure for BF 3. Drawing the Lewis Structure for BF 3. The video covers the basic Lewis structures youll see in an introductory chemistry class.
Drawing the Lewis Structure for PH 3. Follow some steps for drawing the lewis dot structure of SeF4. The drawing of a Lewis Structure is the first step towards predicting the 3-D shape of molecules.
The chlorine atom is situated in the central position of the HClO3 lewis structure since it is the least electronegative atom. Once we know how many valence electrons there are in PH3 we can distribute them around the central atom and attempt to fill the outer shells of each atom. After determining how many valence electrons there are in BF 3 place them around the central atom to complete the octets.

Lewis Structures 5 Steps 1 Count Valence E Available If An Anion Add Charge To Valence E If A Cation Subtract Charge From Valence E 2 Draw Skeleton Ppt Download

Lewis Dot Structure Definition Examples And Drawing

Ppt Drawing Lewis Structures Powerpoint Presentation Free Download Id 529421
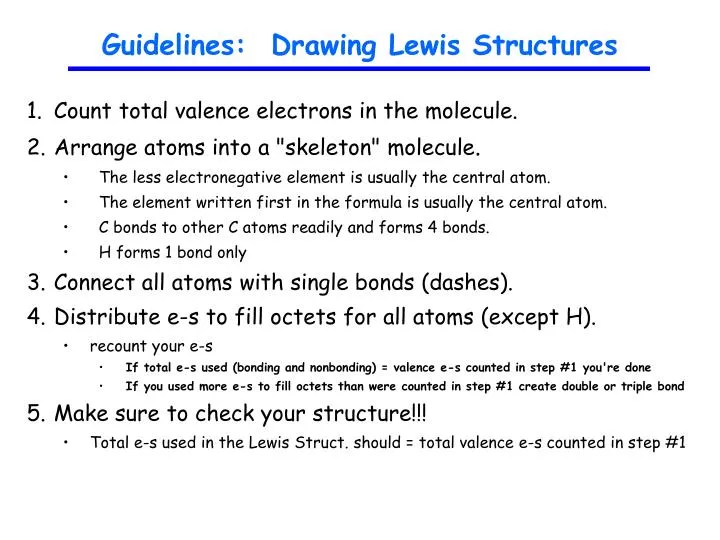
Ppt Guidelines Drawing Lewis Structures Powerpoint Presentation Free Download Id 1812949

Lewis Diagrams Made Easy How To Draw Lewis Dot Structures Youtube

Lewis Structures Made Easy Examples And Tricks For Drawing Lewis Dot Diagrams Of Molecules Youtube

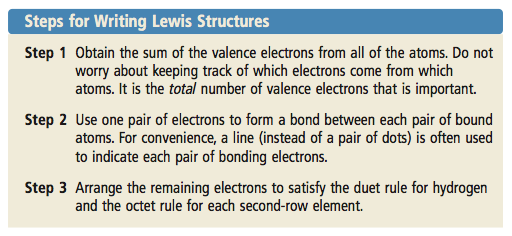
0 comments
Post a Comment